OH MY.. Why Are Empirical Formulas Important In Chemistry
An empirical formula is the formula which we get by dividing the number of ions with the highest common factor. And here we going to investigate how to calculate the empirical formula for any compound and why it is significant.

Empirical And Molecular Formula Notes
As mentioned earlier the empirical formula provides us the information about the least possible ratio of each element in the compound.

Why are empirical formulas important in chemistry. It is easy to find the trends and top topics of why are empirical formulas used in chemistry here. This is not to say that your teacher is a moron. They are written by reducing the subscripts in the molecular formulas to the smallest.
The empirical formula of a substance can be calculated from its percent composition and the molecular formula can be determined from the empirical formula and the compounds molar mass. See full answer below. The empirical formula are the simplest chemical formulas.
What is the empirical formula of the compound. An empirical formula like a molecular formula lacks any structural information about the positioning or bonding of atoms in a molecule. Many compounds tend to have the same empirical formulas as their molecular formulas.
The empirical formula is the simplest whole number ratio defining the ratio of constituent atoms in a species. Learn vocabulary terms and more with flashcards games and other study tools. The people who are in charge of determining what you learn are morons.
The Empirical formula is the lowest whole number ratio of the atoms of the elements in a compound. We can not do the same thing for C2H4 because C2H4 and CH2 are very different. A molecular formula uses chemical symbols and subscripts to indicate the exact numbers of different atoms in a molecule or compound.
The empirical formula of a chemical compound represents the ratio of the elements present in that compound. The percent composition of a. The empirical formula tells us which elements are present and the simplest whole-number ratio of their atoms but not necessarily the actual number of atoms in a given molecule.
The empirical formula of NaCl. It is important to note that the empirical formula does not describe the composition of the molecule. So why are we learning about empirical formulas anyway.
Chemistry is even called the central science because it bridges physics with other natural sciences such as geology and biology. C 2 H 2 both have the same empirical formula of CH. The molecular formula contains information on the actual number of atoms of each element in the molecule where C3H8 or C6H18.
The empirical formula gives information about the ratio of numbers of atoms in the compound. The empirical formula for glucose is CH 2 O. Empirical formulas are important in chemistry because they demonstrate the relationship between the number of each elements atoms in a molecule.
An empirical formula is also called as the stiochiometric formula. And it is approached by experimentthat is what empirical means by experiment. An ionic or a giant covalent compound is described by a simple formula commonly called as the empirical formula.
Empirical formulas show the simplest whole-number ratio of atoms in a compound molecular formulas show the number of each type of atom in a molecule and structural formulas show how the atoms in a molecule are bonded to each other. Ive always been honest with you so its time that you learned the reality of introductory chemistry. It can therefore describe a number of different structures or isomers with varying physical properties.
Echemi supplies various why are empirical formulas used in chemistry news. For butane and isobutane the empirical formula for both molecules is C 2 H 5 and they share. An empirical formula gives the simplest whole-number ratio of atoms in a compound.
In this video we show you how to calculate the empirical. An empirical formula for a compound is the formula of a substance written with the smallest integer subscript. Empirical formula or stiochiometric formula of a substance is the simplest formula which gives the lowest whole-number ratio between the number of atoms of different elements present in the.
It is important because without it we can not define anything for ionic compounds. And given an unknown compound usually an organic compound of the form CxH yN zO the compound can be COMBUSTED in a furnace and the product gases. 1 The first step in this problem is to change the to grams.
Molecular formula n empirical formula. A structural formula indicates the bonding arrangement of the atoms in the molecule. The empirical formula of a substance can be calculated from the experimentally determined percent composition the percentage of each element present in a.
Browse diverse articles and stories on why are empirical formulas important in chemistry. Empirical Formulae can be derived from the molecular formulae. Empirical formulae are usually obtained based on the analysis of experimental data.
What is the formula for empirical formula. Start studying CHEMISTRY Why We Use Chemical Formulas Chemical Formulas Empirical Formula Symbols and Structures. The empirical formula is the simplest version of a chemical formula for example C3H8.
Empirical molecular and structural. There are three main types of chemical formulas. And given the empirical formula and THEN a determination of the molecular mass of the substance we can find the molecular formula which of course is a whole number multiple of the empirical formula.

Chemical Formula Difference Between Empirical Formula And Molecular Formula Chemistry Notes Best Online Free Chemistry Class 9 12
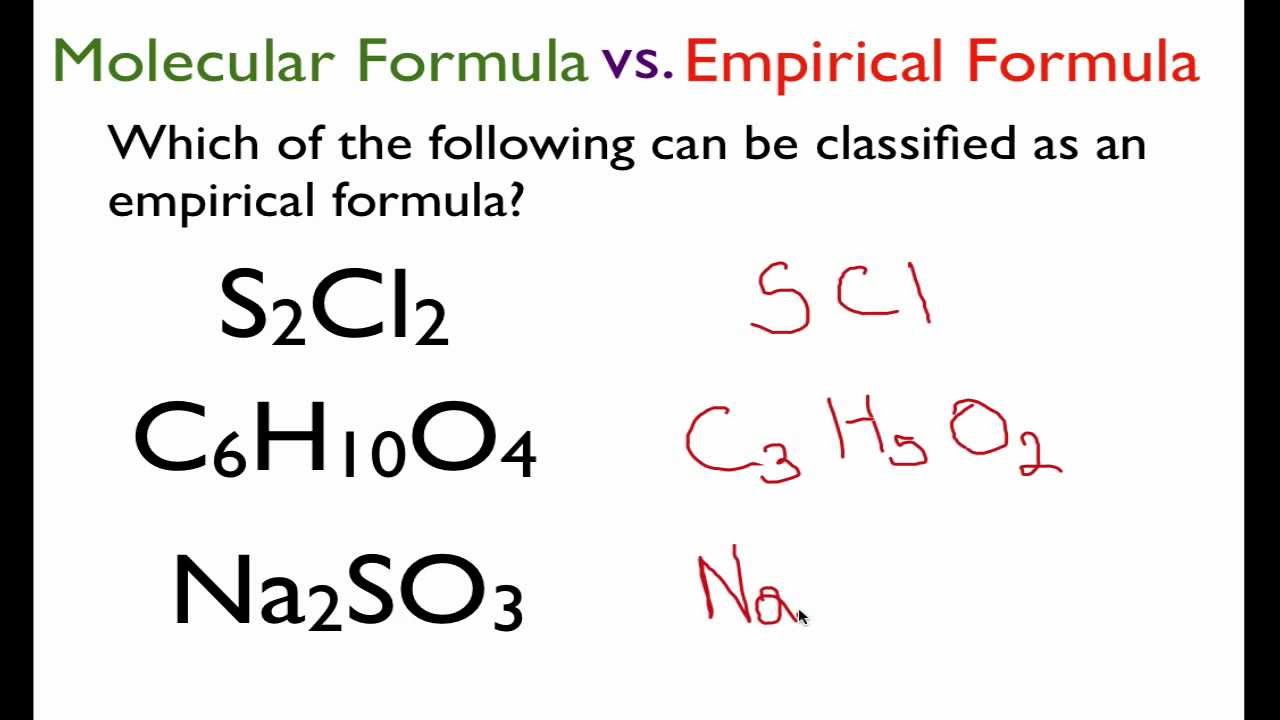
Chemical Formulas Boundless Chemistry

Empirical Formulas Introduction To Chemistry
Molecular Formulas And Nomenclature

Difference Between Empirical And Molecular Formula Infographic Chemistry Basics Chemistry Lessons Chemistry Education

Empirical Formula Definition Steps Examples Video Lesson Transcript Study Com

Writing Empirical Formulas From Percent Composition Combustion Analysis Practice Problems
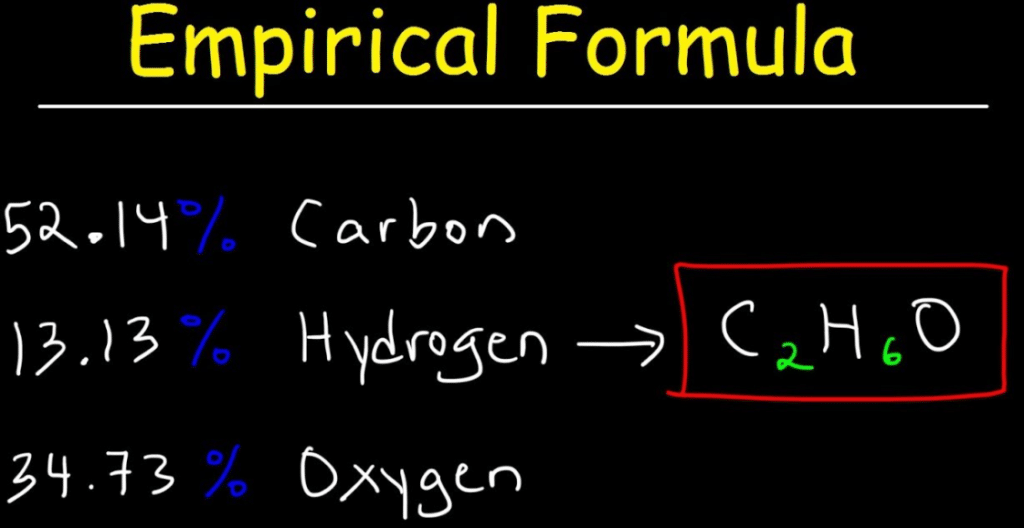
Empirical Formula Definition Get Education
Section 6 5 Emperical Versus Molecular Formulas
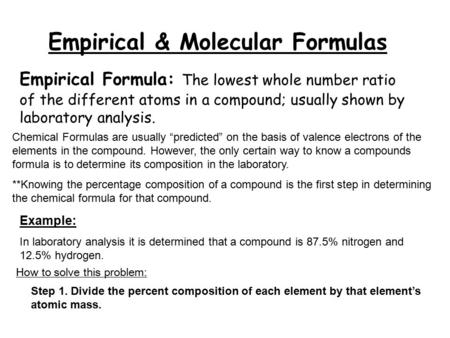
Empirical Formula Molecular Formula Ppt Download

Empirical Formulas From Percents And Mass Empirical Formula Definition A Formula That Shows The Simplest Whole Number Ratio Of The Atoms In A Compound Ppt Download

Chemical Formula Difference Between Empirical Formula And Molecular Formula Chemistry Notes Best Online Free Chemistry Class 9 12

Difference Between Empirical And Molecular Formulas Compare The Difference Between Similar Terms
Molecular Formulas And Nomenclature
Molecular Formulas And Nomenclature

Empirical Molecular And Structural Formulas Video Khan Academy
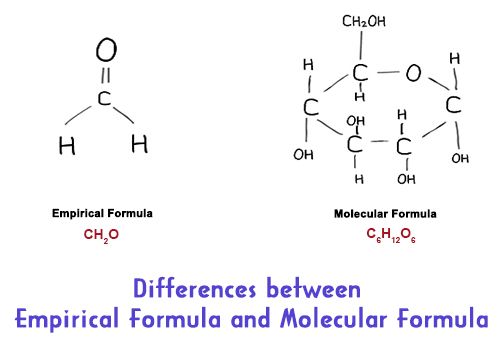
Differences Between Empirical Formula And Molecular Formula Qs Study
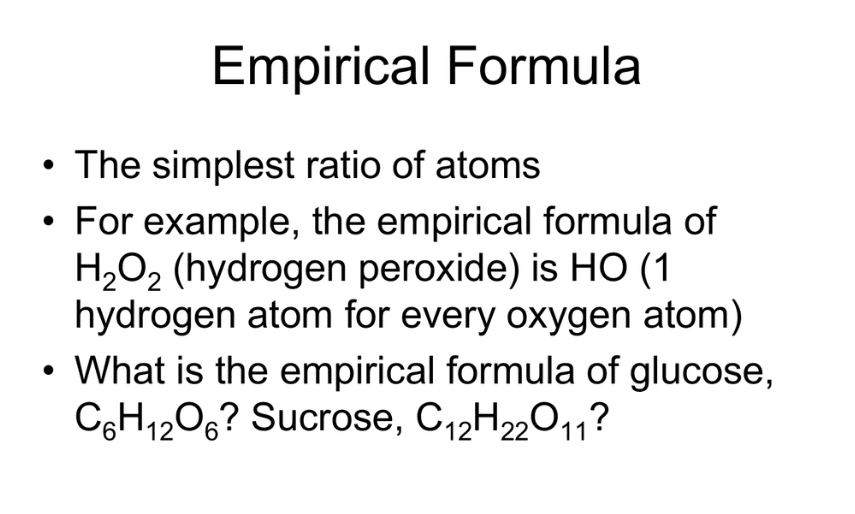
Empirical Formula Definition Get Education
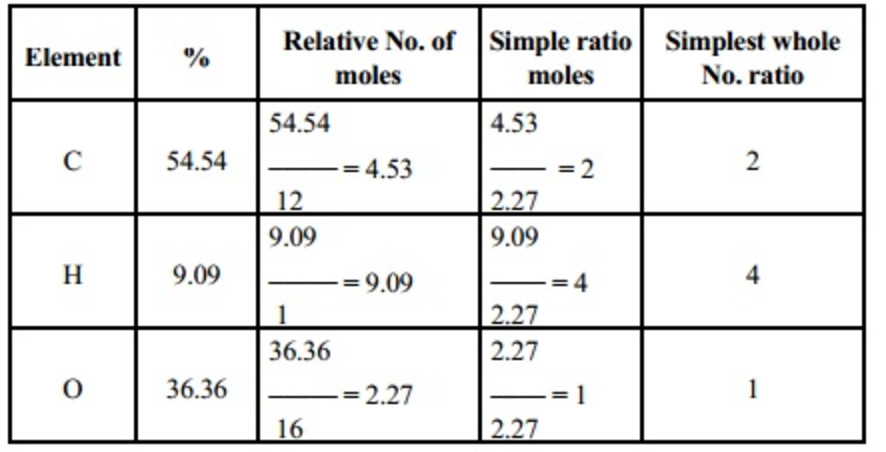
Empirical And Molecular Formula Chemistry Class 11 Some Basic Concepts Of Chemistry
